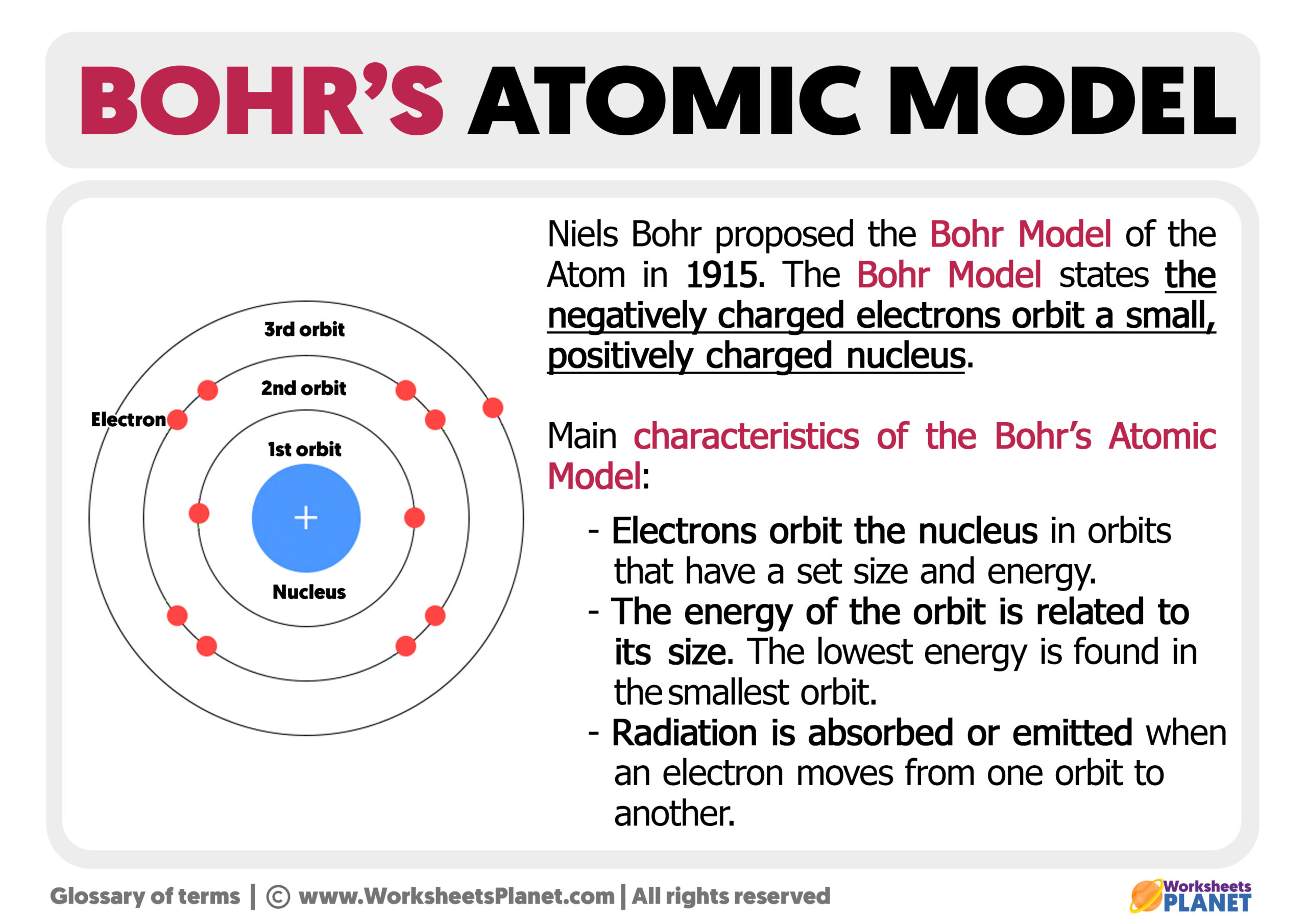
Bohr Model Of Atom Drawbacks . drawbacks of bohr's atomic model: He could not explain the details of the hydrogen and helium atomic spectrum. in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. — bohr’s atomic model became the predecessor of quantum mechanical models. This theory of the atomic model. Learn its history, main postulates, theory, & formula. First, the interaction of multiple atoms makes their. bohr atomic model described & explained with examples, diagrams, & problems. Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel.
from www.worksheetsplanet.com
in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. drawbacks of bohr's atomic model: bohr atomic model described & explained with examples, diagrams, & problems. — bohr’s atomic model became the predecessor of quantum mechanical models. He could not explain the details of the hydrogen and helium atomic spectrum. This theory of the atomic model. Learn its history, main postulates, theory, & formula. First, the interaction of multiple atoms makes their.
Bohr's Atomic Model
Bohr Model Of Atom Drawbacks — bohr’s atomic model became the predecessor of quantum mechanical models. drawbacks of bohr's atomic model: Learn its history, main postulates, theory, & formula. Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. — bohr’s atomic model became the predecessor of quantum mechanical models. He could not explain the details of the hydrogen and helium atomic spectrum. — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. First, the interaction of multiple atoms makes their. This theory of the atomic model. bohr atomic model described & explained with examples, diagrams, & problems. in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,.
From www.chemistrylearner.com
Bohr Model Definition, Features, and Limitations Bohr Model Of Atom Drawbacks This theory of the atomic model. He could not explain the details of the hydrogen and helium atomic spectrum. drawbacks of bohr's atomic model: in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. Learn its history, main postulates, theory, & formula. Bohr model in 1921 [4] after. Bohr Model Of Atom Drawbacks.
From techiescientist.com
Boron Bohr Model Diagram, Steps To Draw Techiescientist Bohr Model Of Atom Drawbacks He could not explain the details of the hydrogen and helium atomic spectrum. Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. bohr atomic model described & explained with examples, diagrams, & problems. drawbacks of bohr's atomic model: This theory of the atomic model. in this article, we will explore. Bohr Model Of Atom Drawbacks.
From www.youtube.com
Defects of Bohr's Atomic Model Bohrs Atomic Theory Drawbacks Bohr Model Of Atom Drawbacks in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. First, the interaction of multiple atoms makes their. — bohr’s atomic model became the predecessor. Bohr Model Of Atom Drawbacks.
From classnotes.org.in
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom Bohr Model Of Atom Drawbacks He could not explain the details of the hydrogen and helium atomic spectrum. bohr atomic model described & explained with examples, diagrams, & problems. First, the interaction of multiple atoms makes their. — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. Learn its history, main. Bohr Model Of Atom Drawbacks.
From learningschoolequalrf.z22.web.core.windows.net
Bohr Atomic Model Explained Bohr Model Of Atom Drawbacks bohr atomic model described & explained with examples, diagrams, & problems. in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. — bohr’s atomic model became the predecessor of quantum mechanical models. First, the interaction of multiple atoms makes their. He could not explain the details of. Bohr Model Of Atom Drawbacks.
From www.studypool.com
SOLUTION Bohr model of atom application and drawback of bohr s model Bohr Model Of Atom Drawbacks — bohr’s atomic model became the predecessor of quantum mechanical models. in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. First, the interaction of multiple atoms makes their. Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. This theory of. Bohr Model Of Atom Drawbacks.
From www.youtube.com
Bohr's Model of Hydrogen atom and it's Drawbacks YouTube Bohr Model Of Atom Drawbacks in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. bohr atomic model described & explained with examples, diagrams, & problems. — bohr’s atomic model became the predecessor of quantum mechanical models. Learn its history, main postulates, theory, & formula. — there are two reasons why. Bohr Model Of Atom Drawbacks.
From www.thephysicsmill.com
Unreal Truths Matter Waves and the Bohr Model of the Atom Bohr Model Of Atom Drawbacks drawbacks of bohr's atomic model: — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. He could not explain the details of the hydrogen and. Bohr Model Of Atom Drawbacks.
From www.expii.com
Bohr's Atomic Model — Overview & Importance Expii Bohr Model Of Atom Drawbacks drawbacks of bohr's atomic model: — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. This theory of the atomic model. He could not explain the details of the hydrogen and helium atomic spectrum. First, the interaction of multiple atoms makes their. Learn its history, main. Bohr Model Of Atom Drawbacks.
From www.youtube.com
Drawbacks of Bohr's Model of Atom Atomic Structure L9 Unacademy Bohr Model Of Atom Drawbacks This theory of the atomic model. in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. bohr atomic model described & explained with examples, diagrams, & problems. Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. drawbacks of bohr's atomic. Bohr Model Of Atom Drawbacks.
From www.youtube.com
Atomic Structure, Calculate Number of Spectral Lines, Drawbacks of Bohr Bohr Model Of Atom Drawbacks First, the interaction of multiple atoms makes their. Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. bohr atomic model described & explained with examples, diagrams, & problems. Learn its history, main postulates, theory, & formula. — bohr’s atomic model became the predecessor of quantum mechanical models. This theory of the. Bohr Model Of Atom Drawbacks.
From www.youtube.com
Bohr's Atomic Model Postulates and drawbacks of Bohr's atomic Model Bohr Model Of Atom Drawbacks — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. He could not explain the details of the hydrogen and helium atomic spectrum. This theory of the atomic model. bohr atomic model described & explained with examples, diagrams, & problems. Bohr model in 1921 [4] after. Bohr Model Of Atom Drawbacks.
From www.youtube.com
CLASS 11 CHEMISTRY II STRUCTURE OF ATOM L9 II Drawbacks of Bohr atomic Bohr Model Of Atom Drawbacks drawbacks of bohr's atomic model: Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. in this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. — there are two reasons why bohr's model doesn't work for atoms with more than one. Bohr Model Of Atom Drawbacks.
From learningschoolequalrf.z22.web.core.windows.net
Describe The Bohr Model Of The Atom Bohr Model Of Atom Drawbacks — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. bohr atomic model described & explained with examples, diagrams, & problems. He could not explain the details of the hydrogen and helium atomic spectrum. drawbacks of bohr's atomic model: First, the interaction of multiple atoms. Bohr Model Of Atom Drawbacks.
From www.nuclear-power.com
Bohr Model of Atom Bohr’s Postulates Definition Bohr Model Of Atom Drawbacks drawbacks of bohr's atomic model: bohr atomic model described & explained with examples, diagrams, & problems. Learn its history, main postulates, theory, & formula. — bohr’s atomic model became the predecessor of quantum mechanical models. Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. He could not explain the details. Bohr Model Of Atom Drawbacks.
From www.thoughtco.com
Bohr Model of the Atom Overview and Examples Bohr Model Of Atom Drawbacks — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. Learn its history, main postulates, theory, & formula. This theory of the atomic model. drawbacks of bohr's atomic model: Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. . Bohr Model Of Atom Drawbacks.
From www.youtube.com
Achievements & Drawbacks of Bohr's Atomic Model CLASS 11 CHEMISTRY Bohr Model Of Atom Drawbacks — there are two reasons why bohr's model doesn't work for atoms with more than one electron, according to the chemistry channel. Learn its history, main postulates, theory, & formula. drawbacks of bohr's atomic model: Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. He could not explain the details of. Bohr Model Of Atom Drawbacks.
From www.pinterest.co.uk
bohr's atomic model of atom Rutherford Model, Model, Bohr Bohr Model Of Atom Drawbacks He could not explain the details of the hydrogen and helium atomic spectrum. drawbacks of bohr's atomic model: Bohr model in 1921 [4] after sommerfeld expansion of 1913 model showing maximum electrons per shell. This theory of the atomic model. First, the interaction of multiple atoms makes their. bohr atomic model described & explained with examples, diagrams, &. Bohr Model Of Atom Drawbacks.